Minerals mining is a huge natural
wealth, where mineral resources are optimally utilized if the will is
essential for the continuity of economic growth. In the belly of this
earth to save countless millions metal content and non-metallic
materials that can be utilized as industrial equipment needs of society
at large. Environmental components that have the potential to support
the development of a mining exploration to create jobs. Where mining
exploration activities require the expertise of trained and skilled
professional and technical personnel who may be widely available in
local communities. In addition to the manpower requirements of mineral
properties and mine development activities often require additional
materials and specialized technical services. It is also often provided
by the company’s geological and mining engineering, who seek office in
the local community to participate in exploration and mine development
contract.
Gold prospectors have won a lot
of wealth and there is a finding that smaller-scale artisanal mining and
managed by local residents. Natural resources are very abundant must be
utilized efficiently and should refer also to the security environment.
Because of environmental aspects will have a major impact on mining.
But it also depends on how where we manage these resources.
In the field of extractive metallurgy, mineral engineering,
mineral processing, also known as mineral dressing or ore dressing, is
the process of separating commercially valuable minerals from their
ores. Mineral processing, treating crude ores and mineral products in
order to separate the valuable minerals from the waste rock, or gangue.
It is the first process that most ores undergo after mining in order to
provide a more concentrated material for the procedures of extractive
metallurgy. The primary operations are comminution and concentration,
but there are other important operations in a modern mineral processing
plant, including sampling , analysis and dewatering
SAMPLING AND ANALYSIS
SAMPLING AND ANALYSIS
Routine sampling and analysis of
the raw material being processed are undertaken in order to acquire
information necessary for the economic appraisal of ores and
concentrates. In addition, modern plants have fully automatic control
systems that conduct in-stream analysis of the material as it is being
processed and make adjustments at any stage in order to produce the
richest possible concentrate at the lowest possible operating cost.
SAMPLING
Sampling is the removal from a given lot of material a portion that
is representative of the whole yet of convenient size for analysis. It
is done either by hand or by machine. Hand sampling is usually
expensive, slow, and inaccurate, so that it is generally applied only
where the material is not suitable for machine sampling (slimy ore, for
example) or where machinery is either not available or too expensive to
install. Many different sampling devices are available, including
shovels, pipe samplers, and automatic machine samplers. For these
sampling machines to provide an accurate representation of the whole
lot, the quantity of a single sample, the total number of samples, and
the kind of samples taken are of decisive importance. A number of
mathematical sampling models have been devised in order to arrive at the
appropriate criteria for sampling
ANALYSIS
After one or more samples are taken from an amount of ore
passing through a material stream such as a conveyor belt, the samples
are reduced to quantities suitable for further analysis. Analytical
methods include chemical, mineralogical, and particle size.
Chemical analysis
Even before the 16th century, comprehensive schemes of assaying
(measuring the value of) ores were known, using procedures that do not
differ materially from those employed in modern times. Although
conventional methods of chemical analysis are used today to detect and
estimate quantities of elements in ores and minerals, they are slow and
not sufficiently accurate, particularly at low concentrations, to be
entirely suitable for process control. As a consequence, to achieve
greater efficiency, sophisticated analytical instrumentation is being
used to an increasing extent.
In emission spectroscopy, an electric discharge is established
between a pair of electrodes, one of which is made of the material being
analyzed. The electric discharge vaporizes a portion of the sample and
excites the elements in the sample to emit characteristic spectra.
Detection and measurement of the wavelengths and intensities of the
emission spectra reveal the identities and concentrations of the
elements in the sample.
Mineralogical analysis
A successful separation of a valuable mineral from its ore can be determined by heavy-liquid testing, in which a single-sized fraction of a ground ore is suspended in a liquid of high specific gravity. Particles of less density than the liquid remain afloat, while denser particles sink. Several different fractions of particles with the same density (and, hence, similar composition) can be produced, and the valuable mineral components can then be determined by chemical analysis or by microscopic analysis of polished sections.
Size analysis
A successful separation of a valuable mineral from its ore can be determined by heavy-liquid testing, in which a single-sized fraction of a ground ore is suspended in a liquid of high specific gravity. Particles of less density than the liquid remain afloat, while denser particles sink. Several different fractions of particles with the same density (and, hence, similar composition) can be produced, and the valuable mineral components can then be determined by chemical analysis or by microscopic analysis of polished sections.
Size analysis
Coarsely ground minerals can be classified according to size by
running them through special sieves or screens, for which various
national and international standards have been accepted. One old
standard (now obsolete) was the Tyler Series, in which wire screens were
identified by mesh size, as measured in wires or openings per inch.
Modern standards now classify sieves according to the size of the
aperture, as measured in millimetres or micrometres (10-6 metre).
Mineral processing can involve four general types of unit operation:
COMMINUTION
In all of these processes, the most important considerations are the economics of the processes and this is dictated by the grade and recovery of the final product. To do this, the mineralogy of the ore needs to be considered as this dictates the amount of liberation required and the processes that can occur. The smaller the particles processes, the greater the theoretical grade and recovery of the final product, but this however is difficult to do with fine particles as they prevent certain concentration processes from occurring.
In order to separate the valuable components of an ore from
the waste rock, the minerals must be liberated from their interlocked
state physically by comminution. As a rule, comminution begins by
crushing the ore to below a certain size and finishes by grinding it
into powder, the ultimate fineness of which depends on the fineness of
dissemination of the desired mineral. Whereas crushing is done mostly
under dry conditions, grinding mills can be operated both dry and wet,
with wet grinding being predominant.
CONCENTRATION
Concentration involves the separation of valuable minerals from the other raw materials received from the grinding mill. In large-scale operations this is accomplished by taking advantage of the different properties of the minerals to be separated. These properties can be colour (optical sorting), density (gravity separation), magnetic or electric (magnetic and electrostatic separation), and physicochemical (flotation separation). There are a number of ways to increase the concentration of the wanted minerals: in any particular case the method chosen will depend on the relative physical and surface chemical properties of the mineral and the gangue. Concentration is defined as the number of moles of a solute in a volume of the solution. In case of mineral processing concentration means the increase of the percentage of the valuable mineral in the concentrate.
Gravity separation is the separation of two or more minerals of different specific gravity by their relative movement in response to the force of gravity and one or more other forces (such as centrifugal forces, magnetic forces, buoyant forces), one of which is resistance to motion (drag force) by a viscous medium such as heavy media, water or, less commonly, air. Gravity separation is one of the oldest technique in mineral processing but has seen a decline in its use since the introduction of methods like flotation, classification, magnetic separation and leaching. Gravity separation dates back to at least 3000 BC when Egyptians used the technique for separation of gold.
It is necessary to determine the suitability of a gravity
concentration process before it is employed for concentration of an ore.
The concentration criterion is commonly used for this purpose, designated  in the following equation (where
in the following equation (where 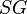 represents specific gravity):
represents specific gravity):
 in the following equation (where
in the following equation (where 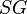 represents specific gravity):
represents specific gravity):- for CC > 2.5, suitable for separation of particles above 75 micron in size
- for 1.75 < CC < 2.5, suitable for separation of particles above 150 micron in size
- for 1.50 < CC < 1.75, suitable for separation of particles above 1.7 mm in size
- for 1.25 < CC < 1.50, suitable for separation of particles above 6.35 mm in size
- for CC < 1.25, not suitable for any size
Gravity methods use the difference in the density of minerals
as the concentrating agent. In heavy-media separation (also called
sink-and-float separation), the medium used is a suspension in water of a
finely ground heavy mineral (such as magnetite or arsenopyrite) or
technical product (such as ferrosilicon). Such a suspension can simulate
a fluid with a higher density than water. When ground ores are fed into
the suspension, the gangue particles, having a lower density, tend to
float and are removed as tailings, whereas the particles of valuable
minerals, having higher density, sink and are also removed. The
magnetite or ferrosilicon can be removed from the tailings by magnetic
separation and recycled.
In the process called jigging, a water stream is pulsed, or moved
by pistons upward and downward, through the material bed. Under the
influence of this oscillating motion, the bed is separated into layers
of different densities, the heaviest concentrate forming the lowest
layer and the lightest product the highest. Important to this process is
a thorough classification of the feed, since particles less than one
millimetre in size cannot be separated by jigging.
Finer-grained particles (from 1 millimetre to 50 micrometres) can
be effectively separated in a flowing stream of water on horizontal or
inclined planes. Most systems employ additional forces—for example,
centrifugal force on spirals or impact forces on shaking tables. Spirals
consist of a vertical spiral channel with an oval cross section. As the
pulp flows from the top to the bottom of the channel, heavier particles
concentrate on the inner side of the stream, where they can be removed
through special openings. Owing to their low energy costs and simplicity
of operation, the use of spirals has increased rapidly. They are
especially effective at concentrating heavy mineral sands and gold ores.
Gravity concentration on inclined planes is carried out on shaking
tables, which can be smoothed or grooved and which are vibrated back and
forth at right angles to the flow of water. As the pulp flows down the
incline, the ground material is stratified into heavy and light layers
in the water; in addition, under the influence of the vibration, the
particles are separated in the impact direction. Shaking tables are
often used for concentrating finely grained ores of tin, tungsten,
niobium, and tantalum.
FROTH FLOTATION
Froth flotation is an important concentration process. This process can be used to separate any two different particles and operated by the surface chemistry of the particles. In flotation, bubbles are introduced into a pulp and the bubbles rise through the pulp. In the process, hydrophobic particles become bound to the surface of the bubbles. The driving force for this attachment is the change in the surface free energy when the attachment occurs. These bubbles rise through the slurry and are collected from the surface. To enable these particles to attach, careful consideration of the chemistry of the pulp needs to be made. These considerations include the pH, Eh and the presence of flotation reagents. The pH is important as it changes the charge of the particles surface and the Eh affects the chemisorption of collectors on the surface of the particles.
Flotation is the most widely used method for the concentration
of fine-grained minerals. It takes advantage of the different
physicochemical surface properties of minerals—in particular, their
wettability, which can be a natural property or one artificially changed
by chemical reagents. By altering the hydrophobic (water-repelling) or
hydrophilic (water-attracting) conditions of their surfaces, mineral
particles suspended in water can be induced to adhere to air bubbles
passing through a flotation cell or to remain in the pulp. The air
bubbles pass to the upper surface of the pulp and form a froth, which,
together with the attached hydrophobic minerals, can be removed. The
tailings, containing the hydrophilic minerals, can be removed from the
bottom of the cell.
The addition of flotation reagents also affects the operation
of these processes. The most important chemical that is added is the
collector, This chemical binds to the surface of the particles as it is a
surfactant. The main considerations in this chemical is the nature of
the head group and the size of the hydrocarbon chain. The hydrocarbon
tail needs to be short to maximize the selectivity of the desired
mineral and the headgroup dictates which minerals it attaches to. The
frothers are another important chemical addition to the pulp at it
enables stable bubbles to be formed. This is important as if the bubble
coalesce, minerals fall off their surface. The bubbles however should
not be too stable as this prevents easy transportation and dewatering of
the concentrate formed. The mechanism of these frothers is not
completely known and further research into their mechanisms is being
performed.
Depressants and activators are used to selectively separate
one mineral from another. Depressants inhibit the flotation of one
mineral or minerals while activators enable the flotation of others.
Examples of these include CN−, used to depress all sulfides but galena
and this depressant is believed to operate by changing the solubility of
chemisorbed and physisorbed collectors on sulfides. This theory
originates from Russia. An example of an activator is Cu2+ ions, used
for the flotation of sphalerite. There are a number of cells able to be
used for the flotation of minerals. these include flotation columns and
mechanical flotation cells. The flotation columns are used for finer
minerals and they typically have a higher grade and lower recovery of
minerals than mechanical flotation cells. The cells in use at the moment
can exceed 300 m3. This is done as they are cheaper per unit volume
than smaller cells, but they are not able to be controlled as easily as
smaller cells.
Flotation makes possible the processing of complex
intergrown ores containing copper, lead, zinc, and pyrite into separate
concentrates and tailings—an impossible task with gravity, magnetic, or
electric separation methods. In the past, these metals were recoverable
only with expensive metallurgical processes.
MAGNETIC SEPARATION
Magnetic separation is a process in which magnetically
susceptible material is extracted from a mixture using a magnetic force.
This separation technique can be useful in mining iron as it is
attracted to a magnet. In this machine the raw ore, after calcination
was fed onto a moving belt which passed underneath two pairs of
electromagnets under which further belts ran at right angles to the feed
belt. The first pair of electromagnets was weakly magnetised and served
to draw off any iron ore present. The second pair were strongly
magnetised and attracted the wolframite, which is weakly magnetic. These
machines were capable of treating 10 tons of ore a day.This process of
separating magnetic substances from the non-magnetic substances in a
mixture with the help of a magnet is called magnetic separation.
Magnetic separation is based on the differing degrees of
attraction exerted on various minerals by magnetic fields. Success
requires that the feed particles fall within a special size spectrum
(0.1 to 1 millimetre). With good results, strongly magnetic minerals
such as magnetite, franklinite, and pyrrhotite can be removed from
gangue minerals by low-intensity magnetic separators. High-intensity
devices can separate oxide iron ores such as limonite and siderite as
well as iron-bearing manganese, titanium, and tungsten ores and
iron-bearing silicates.
This process operates by moving particles in a magnetic
field. The force experienced in the magnetic field is given by the
equation f=m/k.H.dh/dx. with k=magnetic susceptibility, H-magnetic field
strength, and dh/dx being the magnetic field gradient. As seen in this
equation, the separation can be driven in two ways, either through a
gradient in a magnetic field or the strength of a magnetic field. The
different driving forces are used in the different concentrators. These
can be either with water or without. Like the spirals, washwater aids in
the separation of the particles while increases the entrainment of the
gangue in the concentrate.
ELECTROSTATIC SEPARATION
The electrostatic method separates particles of different
electrical charges and, when possible, of different sizes. When
particles of different polarity are brought into an electrical field,
they follow different motion trajectories and can be caught separately.
Electrostatic separation is used in all plants that process heavy
mineral sands bearing zircon, rutile, and monazite. In addition, the
cleaning of special iron ore and cassiterite concentrates as well as the
separation of cassiterite-scheelite ores are conducted by electrostatic
methods.
There are two main types of electrostatic separators. These
work in similar ways, but the forces applied to the particles are
different and these forces are gravity and electrostatic attraction. The
two types are electrodynamic separators (or high tension rollers) or
electrostatic separators. In high tension rollers, particles are charged
by a corona discharge. This charges the particles that subsequently
travel on a drum. The conducting particles lose their charge to the drum
and are removed from the drum with centripetal acceleration.
Electrostatic plate separators work by passing a stream of particles
past a charged anode. The conductors lose electrons to the plate and are
pulled away from the other particles due to the induced attraction to
the anode. These separators are used for particles between 75 and 250
micron and for efficient separation to occur, the particles need to be
dry, have a close size distribution and uniform in shape. Of these
considerations, one of the most important is the water content of the
particles. This is important as a layer of moisture on the particles
will render the non-conductors as conductors as the layer of the water
is conductive.
Electrostatic plate separators are usually used for streams
that have small conductors and coarse non-conductors. The high tension
rollers are usually used for streams that have coarse conductors and
fine non-conductors. These separators are commonly used for separating
mineral sands, an example of one of these mineral processing plants is
the CRL processing plant at Pinkenba in Brisbane Queensland. In this
plant, zircon, rutile and ilmenite are separated from the silica gangue.
In this plant, the separation is performed in a number of stages with
roughers, cleaners, scavengers and recleaners.
DEWATERING
Dewatering is an important process in mineral processing. The purpose of dewatering is to remove water absorbed by the particles which increases the pulp density. This is done for a number of reasons, specifically, to enable ore handling and concentrates to be transported easily, allow further processing to occur and to dispose of the gangue. The water extracted from the ore by dewatering is recirculated for plant operations after being sent to a water treatment plant. The main processes that are used in dewatering include dewatering screens such as Sepro-Sizetec Screens, sedimentation, filtering, and thermal drying. These processes increase in difficulty and cost as the particle size decreases.
Dewatering screens operate by passing particles over a screen.
The particles pass over the screen while the water passes through the
apertures in the screen. This process is only viable for coarse ores
that have a close size distribution as the apertures can allow small
particles to pass through
Sedimentation operates by passing water into a large thickener or
clarifier. In these devices, the particles settle out of the slurry
under the effects of gravity or centripetal forces. These are limited by
the surface chemistry of the particles and the size of the particles.
To aid in the sedimentation process, flocculants and coagulants are
added to reduce the repulsive forces between the particles. This
repulsive force is due to the double layer formed on the surface of the
particles. The flocculants work by binding multiple particles together
while the coagulants work by reducing the thickness of the charged layer
on the outside of the particle.
Thermal drying is usually used for fine particles and to
remove low water content in the particles. Some common processes include
rotary dryers, fluidised beds, spray driers, hearth dryers and rotary
tray dryers. This process is usually expensive to operate due to the
fuel requirement of the dryers.